PAN-TB Priority Candidates







Data from the PAN-TB Collaboration’s 2024 nonclinical studies—covering combination pharmacokinetics, relapsing mouse model (RMM) efficacy, and CFU-R/S ratio mouse model (CRSMM) efficacy—are now available through the Critical Path Institute Data Analytics Platform (CP-DAP).
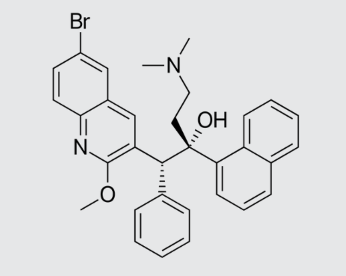
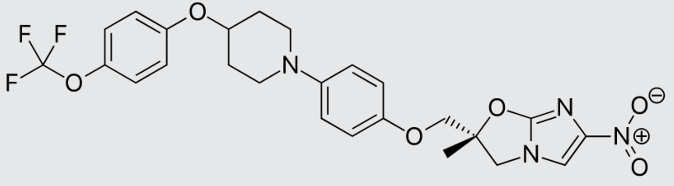
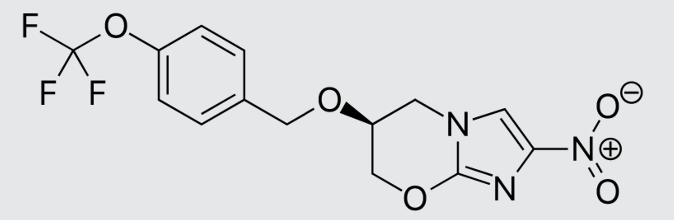
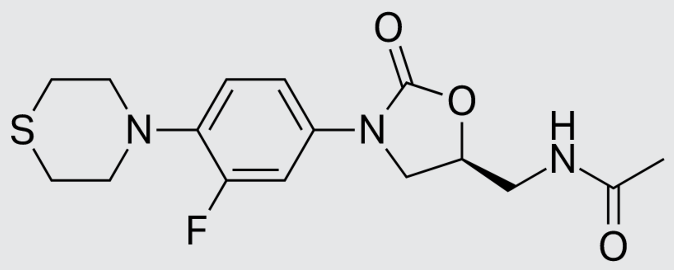
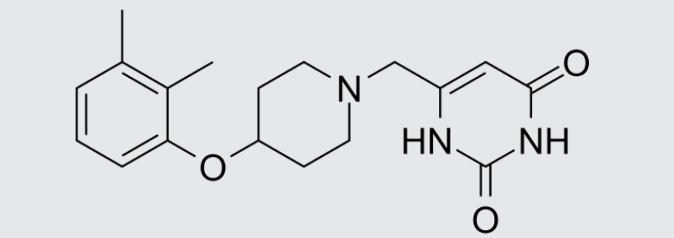
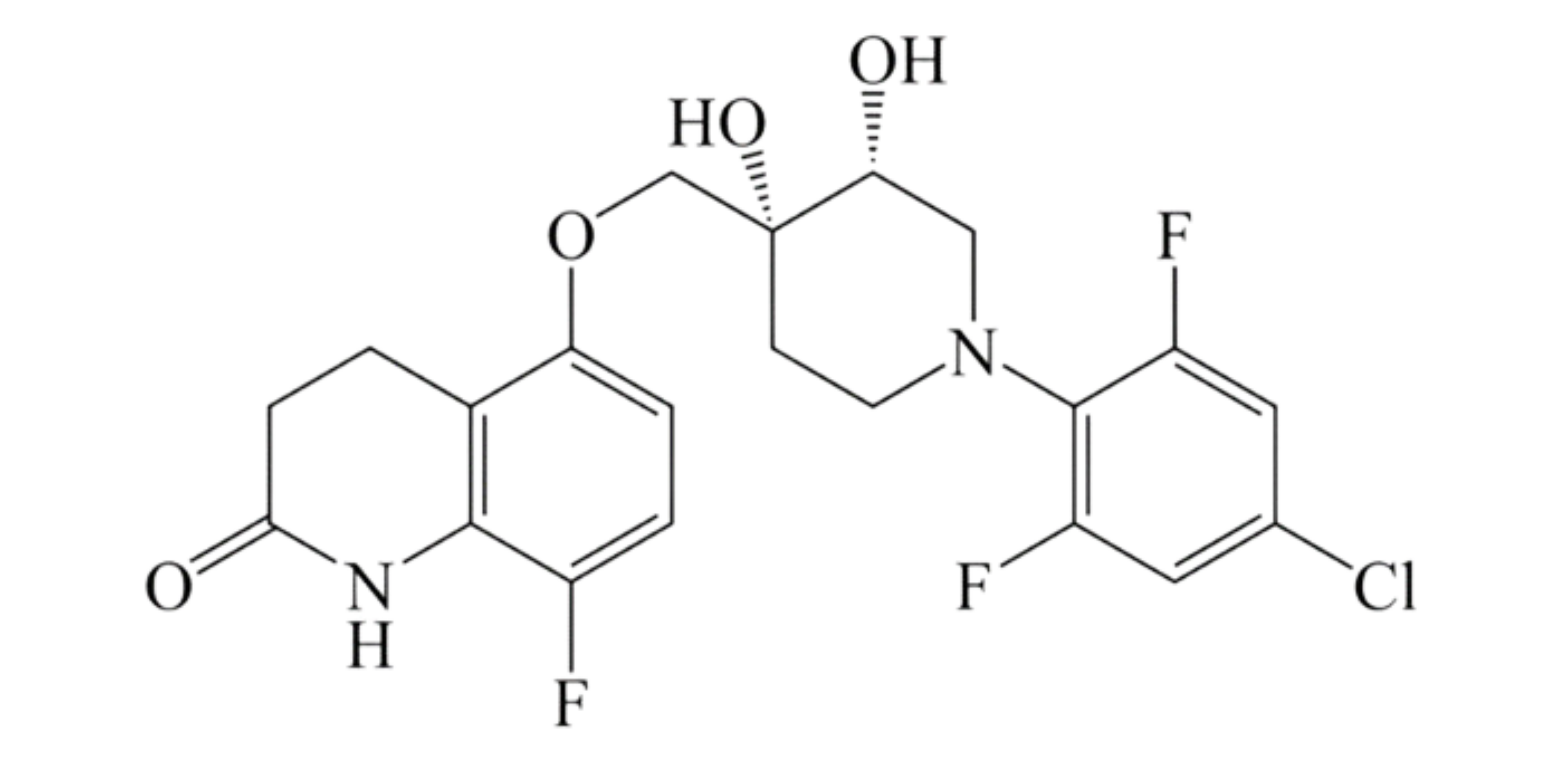
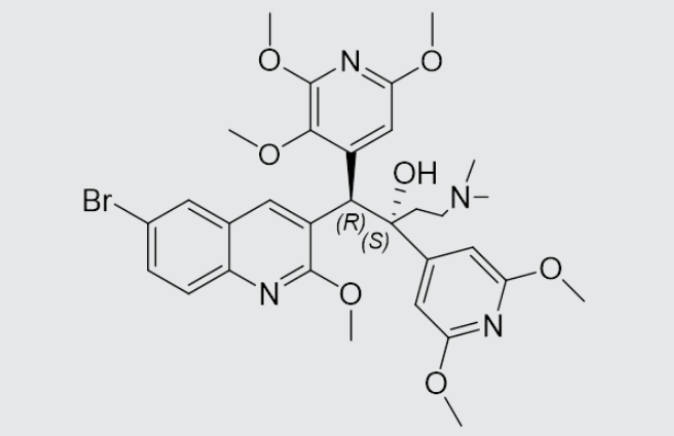
These studies evaluated the pharmacokinetics and efficacy of novel three– and four-drug combinations of the following priority anti-TB drugs and drug candidates: Bedaquiline (B), Sorfequiline (formerly TBAJ-876), Pretomanid (Pa), Sutezolid (Sut), GSK2556286 (286), GSK3036656 (656), TBD09 (formerly MK-7762), TBD11 (formerly mCLB073), Quabodepistat (Q) (formerly OPC167832 or OPC), Rifapentine (P), Isoniazid (H), Pyrazinamide (P) and Moxifloxacin (M).
Study data available:
All available data are mapped to the Clinical Data Interchange Standards Consortium (CDISC) Standard for Exchange of Nonclinical Data (SEND). Please note that C-Path has migrated to a new, enhanced platform, which will provide expanded functionality and capabilities for researchers. All available PAN-TB Collaboration data, including earlier 2020-2023 nonclinical studies, are available through the new platform.
Researchers can request access to the data by following the link below. New data will be added as they become available.


The members of the PAN-TB collaboration have committed to leveraging their unique assets, resources and scientific expertise to advance the development of novel regimens.
























© 2025-2026 Gates Foundation. All rights reserved.
This site is hosted and maintained by the Gates Foundation.